697

3.2. REQUIREMENTS FOR HEAT TREATMENT
PASTEURIZATION OF MILK AND CREAM
Pasteurization of milk in vats and cream in plate pasteurizer
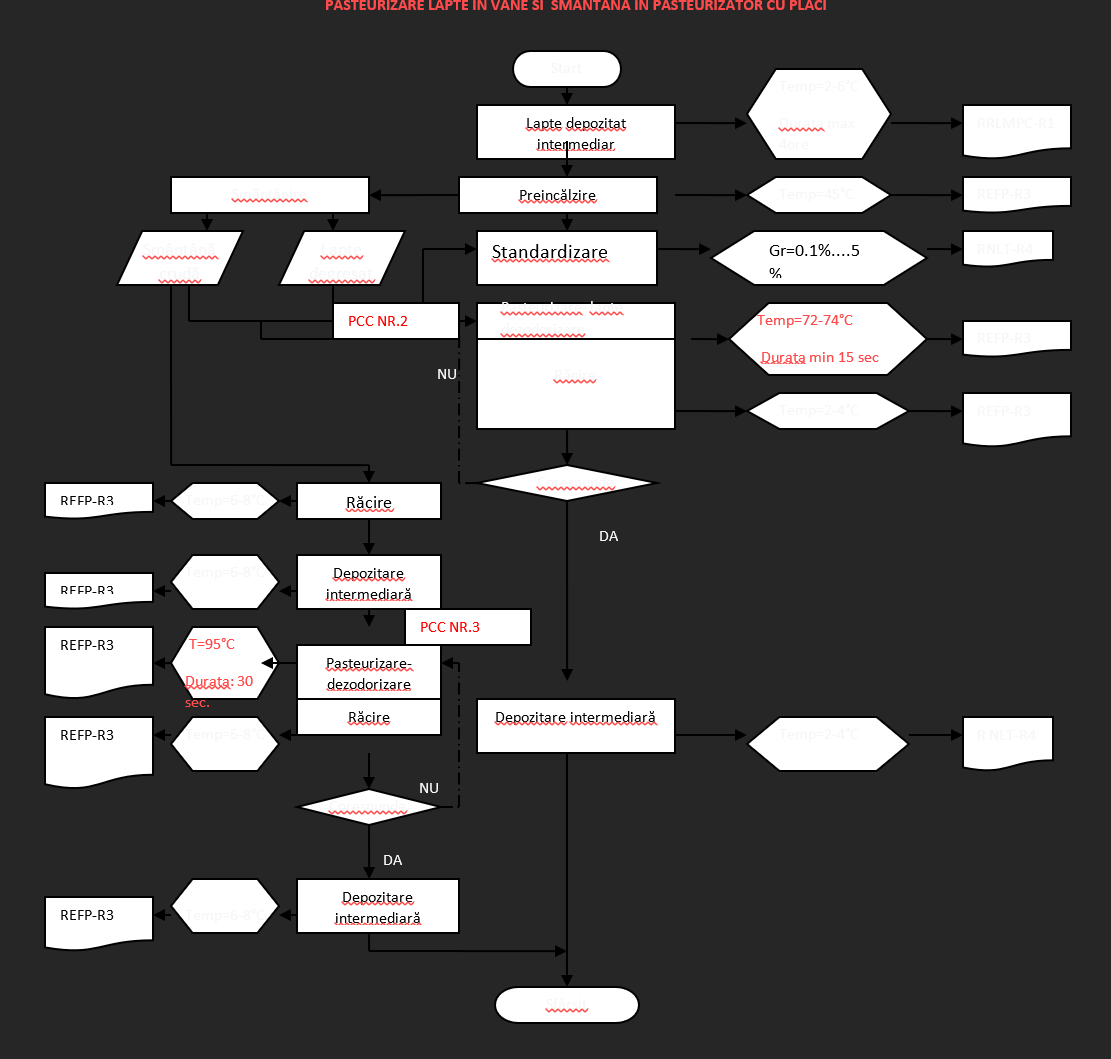
CCP 2 = Critical Control Point – temperature and duration of the thermal process (example)
CRITICAL CONTROL POINT NO. 2
MILK PASTEURIZATION IN VAT Hazard: biological
Operating limit: – Temperature: 85 °C, with a holding time of 30 minutes
Alert limit: – Temperature: 75 °C, with a holding time of 30 seconds
Critical limit: – Temperature: 72 °C, with a holding time of 30 seconds
CCP responsible: – Pasteurization operator
Working method: – Verification of pasteurization temperature and holding time
Frequency: – Continuous, using a temperature logger; spot checks with a thermometer
Documentation: – Electronic, on PC, and manual, in the production logbook
Corrective actions: – Phosphatase test in the laboratory – Re-pasteurization of the milk – In the event of a malfunction, the electrician is notified
Responsibilities: – Pasteurization operator, plant manager, electrician
CRITICAL CONTROL POINT NO. 3
CREAM PASTEURIZATION IN PLATE PASTEURIZER Hazard: biological
Operating limit: – Temperature: 95 °C, with a holding time of 30 seconds
Alert limit: – Temperature: 94 °C, with a holding time of 30 seconds
Critical limit: – Temperature: 92 °C, with a holding time of 30 seconds
CCP responsible: – Pasteurization operator
Working method: – Verification of pasteurization temperature and holding time
Frequency: – Continuous, using a temperature logger; spot checks with a thermometer
Documentation: – Electronic, on PC, and manual, in the production logbook
Corrective actions: – Laboratory testing – Re-pasteurization of the product – In the event of a malfunction, the electrician is notified
Responsibilities: – Pasteurization operator, plant manager, electrician
APPLICABLE LEGISLATION
Regulation (EC) No. 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin (OJ L 139, 30.4.2004, p. 55)
Relevant provisions
When raw milk, colostrum, dairy products or colostrum-based products are subjected to heat treatment, food business operators must ensure compliance with the requirements set out in Regulation (EC) No. 852/2004, Annex II, Chapter XI.
In particular, it must be ensured that, when the following procedures are used, they comply with the specifications below:
(a) Pasteurization, carried out by a treatment involving: (i) high temperature for a short time (at least 72 °C for 15 seconds); (ii) low temperature for a longer time (at least 63 °C for 30 minutes); or (iii) any other equivalent combination of time and temperature, such that the products show a negative reaction to the phosphatase test immediately after treatment.
(b) UHT treatment, carried out by a process: (i) involving a continuous flow of heat at high temperature (minimum 135 °C, with an appropriate holding time), so that no viable microorganisms or spores are able to develop in the treated product stored aseptically; (ii) sufficient to guarantee the microbiological stability of the product for 15 days at 30 °C.
Food business operators must: (a) apply procedures based on the HACCP principles; (b) comply with the requirements established by the competent authority.
CRITERIA APPLICABLE TO RAW COW’S MILK
Operators must ensure that: (a) raw milk used for the manufacture of dairy products has a total bacterial count of < 300,000/ml at 30 °C; (b) heat-treated milk has a total bacterial count of < 100,000/ml at 30 °C.
Where the criteria are not met, operators must inform the competent authority and take corrective measures.
OBSERVATIONS
The use, through marketing means, labels or other media, of claims such as “gentle pasteurization”, “mild”, etc., under the conditions in which:
– standard pasteurization treatments at 129–135 °C, with a holding time of 3–5 seconds, are used for ESL milk with a shelf life of 3–4 weeks; – standard treatments are used for milk intended for fresh products and cream, of at least 65 °C for 30 minutes or 85–90 °C for a minimum of 30 minutes (in vat) or 15 seconds (in plate pasteurizer);
constitutes a misleading claim for the consumer.
P.S. For all the observations and texts above, there is extensive documentation in the scientific literature, technological textbooks, as well as photo-video materials demonstrating marketing exaggerations and denigration of competitors, practices prohibited by Law No. 363/2007 on combating unfair commercial practices.
Dr. Eng. George Grecu





