988
As concerns related to African swine fever (ASFv) continue to significantly impact the pork industry, it can sometimes be challenging to interpret and reconcile conflicting research findings. A recent example in this regard involves separate studies regarding the temperature required for ASFv inactivation to prevent transmission, as reported by PigProgress.
2 studies, 2 different conclusions on ASFv inactivation
Time and temperature are currently the most effective ways to deactivate ASFv. However, different methods of assessing time and temperature can lead to different results.
In 2023, the University of Minnesota (UMN) released preprinted data indicating that two mega-double-stranded DNA viruses, Emiliania huxleyi virus (EhV) and ASFv, were damaged by heat treatment but remained potentially viable when exposed to temperatures of up to 100°C for 20 minutes.
However, in a journal article published in Pathogens, also in 2023, the German ASFv reference laboratory reported that 59°C effectively eliminated ASFv infectivity.
Why do these two studies have very different conclusions? The main difference lies in the methods used to assess the potential infectious capacity of ASFv subjected to different heat treatments.
UMN: Viable PCR method
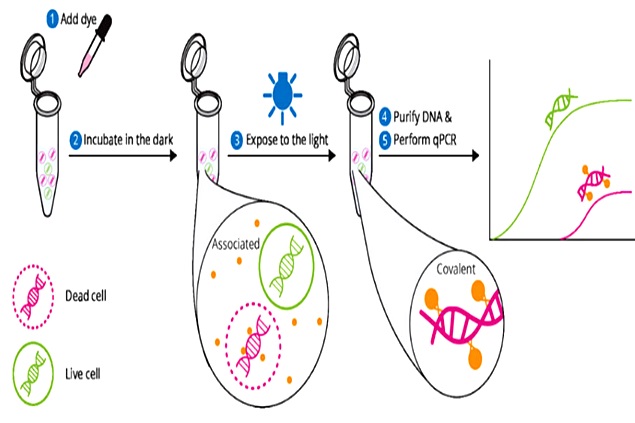
UMN employed a "viable" PCR method, which utilizes a special dye to penetrate the membranes of heat-treated mega-viruses (see Figure 1). The theory is that the dye can penetrate the damaged membrane and bind to the DNA genome, thus interfering with PCR replication.
However, the dye does not penetrate undamaged membranes. Therefore, the viable PCR method described by UMN can distinguish between intact membrane virus particles and damaged membrane virus particles, which is an advantage over standard PCR methods that detect only the genome regardless of membrane damage.
No further replication?
The discovery of limited dye penetration in both EhV and ASFv, even when exposed to temperatures of up to 100°C for 20 minutes, is an intriguing observation that requires further research. However, this does not necessarily mean that the virus particles are still capable of causing infection.
Even if the dye does not penetrate undamaged membranes, it does not mean that the treatment did not damage the virus genome and render it incapable of further replication. For example, exposure to ultraviolet light (UV-C) damages genome cross-linking, making the virus unable to replicate, but not necessarily harming the virus membrane.
This aspect of the viable PCR method equating dye penetration resistance with the virus particle's ability to infect an animal cell has not been scientifically validated for ASFv. In fact, in the same UMN publication, the authors also evaluated EhV infectivity in cell culture, and exposure to 50°C and 60°C was sufficient to eliminate viral infection within 30 seconds.
German ASFv reference laboratory
The German ASFv reference laboratory used the widely accepted historical method to examine ASFv infectivity after the composting of infected wild boar carcasses. This approach is a scientifically validated method for assessing ASFv infectivity using a virus isolation method with macrophages derived from porcine peripheral blood.
The primary mechanism of ASFv infection is through phagocytosis by porcine macrophage cells. Exposing ASFv to their target macrophage cells in culture provides phagocytosis conditions that mimic natural infection.
The study showed that ASFv was no longer infectious once the compost pile temperature reached 59°C, although the ASFv genome using the standard PCR method remained present, once again confirming that standard PCR methods do not differentiate between viable and non-viable virus.
The thermal inactivation data from the German ASFv reference laboratory confirm previous study findings that 60°C for 30 minutes and 60°C for 15-20 minutes were sufficient to inactivate ASFv infectivity. Additionally, EU Directive 2002/99/EC recognizes that heat treatment at a minimum temperature of 80°C is sufficient to inactivate ASFv in meat.
Potential viable genome vs. infectivity
A potential viable genome does not equate to infectivity. It is well-known that DNA is highly stable at temperatures below 100°C. PCR tests rely on DNA remaining stable at 95°C. Therefore, it is not surprising that PCR tests can detect the ASFv genome even after heat treatment at 100°C.
The limited penetration of the dye even when both EhV and ASFv were exposed to temperatures of up to 100°C for 20 minutes is a very interesting finding. These results suggest that a viable genome could potentially remain even with high-temperature treatment.
In fact, both EhV and ASFv lost infectivity with heat treatments at 50°C and 60°C, as reported by both UMN and the German reference laboratory, as well as in previous publications. Both studies demonstrated that heat treatments were effective in reducing infectivity. Other methods, such as viral exposure to UV-C, could provide additional assurance for inactivating resistant viruses like ASFv.
Further development and scientific validation are necessary.