1374

In recent years, milk processing technology has made significant progress, particularly in terms of preserving the main constituents of milk in their most unaltered form:
Food ID: 6 Sødmælk, konventionel (ikke-økologisk) – Danish Name: Milk, whole, conventional (not organic), 3.5% fat Taxonomic name: Bos taurus (Linnaeus, 1758)
Even though there are companies that, in their marketing activity, come up with all sorts of ideas, innovations, exaggerations, diversions, or inaccuracies (“artisanal–artisan,” “gentle” pasteurization – ESL type, but at 80°C – or perhaps at 129°C! – without homogenization, “fantastic” cultures, “gentle” processing, “only natural,” and so on), the technology itself is applied by everyone in a nearly uniform manner, depending on several parameters:
- the available equipment – older, newer, automated, semi-automated, or manual;
- the technological flows and their observance in accordance with legislation;
- the FSSC 22000 and IFS Food quality systems, in their latest versions, correctly implemented throughout the year;
- the operating personnel working with the installed equipment;
- hygiene and compliance with HACCP principles;
- the management staff, both in terms of technological and technical expertise and managerial capability;
- top management involvement and the allocation of necessary resources;
- adherence to product standards developed over the years that have become, to some extent, quality indicators (NTR, STR, SP, SR, etc. – even if Romanian legislation no longer requires their mandatory observance) and the elimination of certain so-called marketing and promotional “gimmicks” used for some products (already familiar to consumers), whereby quality defects defined in the standards are presented as major achievements on packaging (colors, “fat plug,” fat percentage or fat/DM ratio differing from standards, various dry matter contents, etc.);
- others.
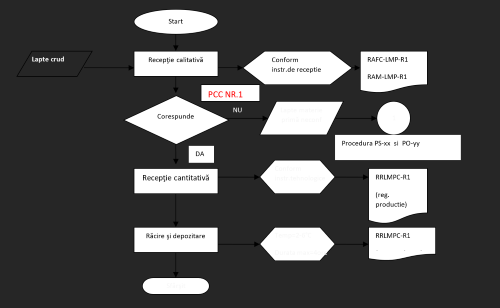
PCC1 = CRITICAL CONTROL POINT = ANTIBIOTICS
R = TECHNOLOGICAL RECORDS PS = SYSTEM PROCEDURE No. xx
PO = OPERATIONAL PROCEDURE No. yy
APPLICABLE LEGISLATION
http://data.europa.eu/eli/reg/2004/853/oj/ron (OJ L 139, 30.4.2004, p. 55)
REGULATION (EC) No 853/2004 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 April 2004 laying down specific hygiene rules for food of animal origin
SECTION IX: RAW MILK, COLOSTRUM, DAIRY PRODUCTS, AND COLOSTRUM-BASED PRODUCTS
3.
(a) Food business operators shall implement procedures to ensure that raw milk meets the following criteria:
(i) For raw cow’s milk: Germ count at 30°C (per ml) Somatic cell count (per ml) ≤ 100,000 (*) ≤ 400,000 (**)
(*) Rolling geometric mean over a period of two months, with at least two samples taken per month. (**) Rolling geometric mean over a period of three months, with at least one sample taken per month, unless the competent authority defines another methodology to take account of seasonal variations in production levels.
(ii) For raw milk from other species: Germ count at 30°C (per ml) ≤ 1,500,000 (*)
(*) Rolling geometric mean over a period of two months, with at least two samples taken per month.
(b) However, when raw milk from species other than cows is intended for the manufacture of products made from raw milk by a process that does not involve any heat treatment, food business operators must ensure that the raw milk meets the following criteria: Germ count at 30°C ≤ 500,000 (*)
(*) Rolling geometric mean over a period of two months, with at least two samples taken per month.
4.
Without prejudice to Directive 96/23/EC, food business operators shall implement procedures to prevent the placing on the market of raw milk:
(a) whose content of antibiotic residues exceeds the permitted level for any of the substances listed in Annexes I and III to Regulation (EEC) No 2377/90;
or
(b) where the total combined residues of all antibiotic substances exceed a maximum permitted value.
If raw milk does not comply with the provisions of points 3 or 4, food business operators shall inform the competent authority and take measures to remedy the situation.
B. Hygiene during milking, collection, and transport
- Milking shall be carried out under hygienic conditions, ensuring in particular that:
(a) before milking begins, the udder and adjacent parts are clean; (b) the milk and colostrum from each animal are checked by the person responsible for milking or by a method yielding equivalent results, to detect abnormal organoleptic or physico-chemical characteristics, and that milk and colostrum presenting such characteristics are not used for human consumption; (c) milk and colostrum from animals showing clinical signs of a disease that may affect the udder are not used for human consumption, unless permitted under a veterinarian’s instructions; (d) animals undergoing treatment that may result in medicinal residues in milk are identified, and milk from such animals, prior to the end of the prescribed withdrawal period, is not used for human consumption; (e) udder treatments applied by immersion or spraying are used in accordance with the procedures laid down in Directive 98/8/EC of the European Parliament and of the Council of 16 February 1998 concerning the placing of biocidal products on the market; (f) colostrum is milked separately and is not mixed with raw milk.
- Immediately after milking, milk and colostrum shall be placed in a clean location designed and equipped to avoid any contamination.
(a) Milk must be rapidly cooled to a temperature not exceeding 8 °C when collected daily, and 6 °C when collection is not daily. (b) Colostrum must be rapidly cooled and stored separately at a temperature not exceeding 8 °C when collected daily, and 6 °C when collection is not daily, or it must be frozen.
During transport, the cooling temperature shall be maintained, and upon arrival at the destination establishment, the milk temperature shall not exceed 10 °C.
Food business operators are not required to comply with the temperature requirements set out in points 2 and 3 if the milk does not meet the criteria in Part III and if: (a) the milk is processed within two hours after milking; or (b) a higher temperature is required for technological reasons related to the manufacture of certain dairy products and the competent authority authorizes it.
The following steps in milk processing – as raw material – will be presented in subsequent chapters. For editorial space reasons, this section on milk processing – raw material – will be divided into several separate articles.
Dr. Eng. George Grecu





